YOU HAVE CHOSEN THE ELEMENT:

ANTIMONY
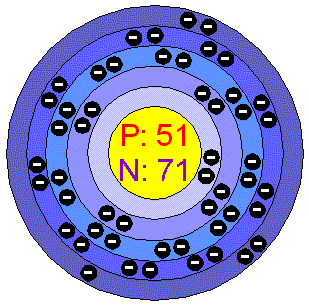
When the ancient citizens discovered Antimony, they were finding an extraordinary element that would be used in several futuristic items. But it wasn't until Basil Valentine, the German alchemist actually discovered it in circa 1450 AD that Antimony was used. Antimony is the 51st element on the periodic table. Its features include an atomic weight of 121.75 grams, a boiling point of 1750 degrees Celsius, and a melting point of 630 degrees Celsius.
Antimony is most commonly found as a bluish-white, brittle, semi-metallic element. It is most commonly found in the Earth's crust (crustal rock). It is not normally found free in natural, and usually associates with silver, arsenic, or bismuth. This substance is most commonly mined in countries such as China, France, Italy, Japan, Mexico, and, on a small scale, the western United States. Actually, Antimony is a common by-product of Copper and Lead ore refining.
Another state which Antimony can exist in is that of a liquid. Liquid Antimony expands as it cools, much in the same way water does. This property makes Antimony useful in making types and patterns of metal. It also acts as a constituent of many other alloys, such as Britannia metal, pewter, Babbitt metal, and Antimonial lead.
Antimony is used in several products. It can form compounds to medicinal agents, safety matches, vulcanized rubber, glass of antimony, yellow pigment for glass and porcelain, butter of antimony, steel bronzing agent, and as a dye.
Antimony is found everywhere you look. It is in our windows, our food, our medicine, and in several other places. This element gives us a great deal of items. Antimony serves the world all around us.
BELOW IS A PICTURE OF ANTIMONY AND IT'S ELECTRON CONFIGURATION