YOU HAVE CHOSEN THE ELEMENT:

ARSENIC
Discovered in compounds dating back to ancient times, Arsenic was discovered by Theophrastus in circa 370-287 BC However, the discovery of Arsenic is generally credited to Albertus Magnus, a Arsenic, atomic symbol As, fills the 33rd spot on the modern periodic table. It is a metallic chemical element, and is used for several different things.
Arsenic has an atomic weight of 74.9216 grams. It melts at 814 degrees Celsius and can be boiled at a temperature of 615 degrees Celsius. These facts give Arsenic its ability to "harden" or re-enforce the alloys it creates.
Arsenic is widely recognized for its role as a poison. But it also can form sulfides, aresenides, arsenites, and arsenates. These forms of Arsenic are used in many industrial reactions, mainly in France, Mexico, Sweden, and the United States.
Arsenic's main use is that of a constituent of alloys. Arsenic readily combines with copper-based alloys to produce temperature-withstanding alloys. This is mainly used in machinery in which the specific parts heat up to high temperatures. Another use of Arsenic in alloys is to re-enforce them. Arsenic, combined with certain elements, creates a stronger alloy.
Arsenic is also widely used in medicines to treat certain types of skin diseases and organ problems. By a strange twist, Arsenic is used in poisons such as pesticides and rat poison. In addition to these uses, Arsenic is used to treat and maintain animal hides and furs, and is used to maintain specimens as well.
Arsenic is more than just a poison. It aids in curing the sick, maintaining specimens and machinery, and creating a resistance to certain conditions. Arsenic's use by the world is growing all the time, and as each day passes, so to does the uses of Arsenic change.
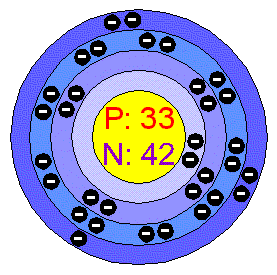
BELOW IS A PICTURE OF ARSENIC'S ELECTRON CONFIGURATION