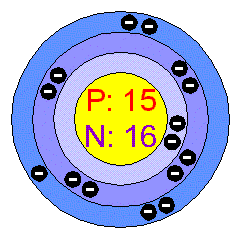
YOU HAVE CHOSEN THE ELEMENT:

PHOSPHORUS
Phosphorus, the 15th element on the periodic table, is second in the Nitrogen group. Phosphorus' atomic weight is given as 30.974 grams. It has been around since 1669, founded by the German alchemist Hennig Brand in the course of experiments in which he attempted to prepare gold from silver. By heating white sand mixed with some evaporated urine, Brand obtained a white solid that glowed in the dark and ignited spontaneously in air. However, it wasn't until 1771 that Karl Wilhelm Scheele prepared it in a larger quantity by heating Calcium Phosphate mixed with Carbon and sand. In the present, the largest source of Phosphorus comes from Phosphate rock.
Phosphorus exists as three different and unique states. First is the ordinary (or white) form, which is quite reactive, and yet is soft enough to be cut with an ordinary knife. This form of Phosphorus is insoluble when put into water. However, Phosphorus must be stored in water or it spontaneously ignites. White Phosphorus is soluble in Carbon di-sulfide and turpentine. White Phosphorus is also highly poisonous, and should be handled with great care.
The second form of Phosphorus takes a reddish color in appearance, and is insoluble to both Carbon di-sulfide and water. This form will not melt under any circumstances and can be ignited with temperatures of 240 degrees Celsius at minimum. Red Phosphorus has a density factor of 2.2 grams per C.C. The third, and rarely used, form of Phosphorus exists in a blackened state. It has a flaky, crystalline texture to it, almost resembling graphite.
Phosphorus is widely distributed in nature, creating .12% of the Earth's crust. Also, a human skeleton is 1400 grams (3.1 pounds) of Phosphorus, while the muscles and the brain nerves are composed of 130 grams and 12 grams, respectively. Phosphorus is a chief source of fertilizers and other products. It is found in such items as detergents, pharmaceuticals, safety matches, and water-softening agents.
Phosphorus is found in many modern things which we use every day. Thanks to Hennig Brand's discovery, modern life is aided by this element and the compounds it can form. The discovery of Phosphorus has truly helped mankind.
BELOW IS A PICTURE OF PHOSPHORUS AND IT'S ELECTRON CONFIGURATION